Cusabio Sus scrofa Recombinant
Abstract
Trefoil factor 3 (TFF3) is involved in cell adhesion, motility, and apoptosis regulates mucosal immunity and maintains the functional integrity of the intestinal epithelium. The upregulation of TFF3 expression in the intestine of newly weaned rats attracted our interest. The present study hypothesized that TFF3 may play a role in preventing diarrhoea in weaned piglets, which is an important consideration in the pig farming industry.
Previous expression yields of recombinant TFF3 protein obtained from Escherichia coli were too low and the bioactivity of the protein was poor. Therefore, this expression system was not suitable for industrial applications. The present study explored the production of recombinant sus scrofa TFF3 in a Brevibacillus Chinensis (B. choshinensis) expression system, with the aim of improving the expression level of the bioactive protein.
To achieve this, the gene fragment encoding sus scrofa recombinant TFF3 was fused into an E. coli-Brevibacillus shuttle vector pNCMO2. High levels of TFF3 (30 mg/L) were produced and secreted into B. Chinensis culture medium in a soluble form with a molecular mass of 13.6 kDa and high immunoreactivity on Western Blot. Therefore, Brevibacillus can be used to produce mucosal factors useful for biochemical analysis and mucosal protection, and in industrial applications to produce new inhibitors of diarrhoea.
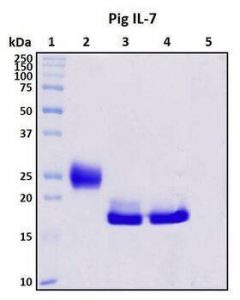
Purity: > 95% as determined by SDS-PAGE
Endotoxin: Please contact us for more information.
Activity
1. Measured by its binding capacity in a functional ELISA. Sus scrofa (pig) immobilized IL10 (Cat: 62000-WNAE) at 2 μg/ml (100 μl/well) can bind to Rhesus IL10RA-HFC (Cat: 90125-C02H), the EC50 of Rhesus IL10RA-HFC is 25- 100ng/mL.
2. Measured in a cell proliferation assay using MC/9-2 mouse mast cells. The ED50 for this effect is typically 1 to 5 ng/mL.
Protein Construction
A DNA sequence encoding porcine IL10 (Q29055) (Ser19-Asn175) was expressed with an initial Met at the N-terminus.
Accession number: Q29055
Expressed Host: E. coli
Species: Sus scrofa (Pig)
Expected N Terminal: Met
Molecular mass
Recombinant porcine IL10 consists of 158 amino acids and has a predicted molecular mass of 18.2 kDa. Its apparent molecular mass is approximately 18 kDa on SDS-PAGE under reducing conditions.
Formulation:
- Lyophilized from sterile PBS, pH 7.4.
- Please contact us with any concerns or special requirements.
- Typically 5%-8% trehalose, mannitol and 0.01% Tween80 are added as protectants prior to lyophilization.
- Please refer to the CoA printout for specific buffer information.
Shipping
Recombinant proteins are generally provided as a lyophilized powder that is shipped at room temperature. Recombinant protein bulk packs are provided as a frozen liquid. They are shipped with blue ice unless customers require otherwise.
Stability and Storage
Samples are stable for up to twelve months from the date of receipt at -20℃ to -80℃. Store it under sterile conditions between -20℃ and -80℃. It is recommended to divide the protein into aliquots for optimal storage. Avoid repeated cycles of freezing and thawing.
Reconstitution
A printed copy of the COA with instructions for reconstitution is shipped with the products. Please refer to it for detailed information.
Materials and methods
- Bacteria, plasmids and media
B. choshinensis strain HB116 (Takara Bio, Inc.) was used in this study. Competent E. coli DH5α cells (Sangon Biotech Co, Ltd.) were used for DNA manipulation. pNCMO2 (Takara Bio, Inc.) and pMD19-T (Takara Bio, Inc.) were used as the vector and subcloning plasmid, respectively. Milk-Tween (MT) medium containing 2% yeast extract, 10% glucose, 10% polypeptide, 5% meat extract, 0.01% FeSO4·7H2O, 0.001% ZnSO4·6H2O was used and 0.01% MnSO4·4H2O to grow strain HB116. E. coli DH5α cells were grown in Luria Broth medium (Oxoid; Thermo Fisher Scientific, Inc.). NaOH was used to adjust the pH of all media to 7.0. Neomycin (20 µg/ml; Beijing Solarbio Science & Technology Co, Ltd.) was added to the medium used to culture bacteria containing pNCMO2 and derivatives.
- RNA extraction and PCR
Total RNA from sus scrofa spleen tissue (preserved in our laboratory) was isolated using a TRIzol® reagent kit (Takara Bio, Inc.) according to the manufacturer’s instructions. cDNA synthesis was performed using a PrimeScript Reverse Transcriptase kit (Takara Bio, Inc.). Primers for the sus scrofa TFF3 gene were designed using Primer v.5.0 software (Sangon Biotech. Co. Ltd.), according to the gene sequence in the GenBank database (accession number NM_001243483).
The mRNA-specific primers (Sangon Biotech Co, Ltd.) were: TFF3 forward, 5′-GCATGGAGGCCAGGATGT-3′ and reverse, 5′-CGGTTAGAAGGTGCATTCT-3′. The PCR program to amplify the TFF3 gene from the cDNA was 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s, 55 °C for 20 s, and 72 °C for 30 s with a final extension step. at 72 °C for 10 min. The PCR products were separated by 2% agarose gel electrophoresis.
Purified PCR fragments were recovered using a PCR gel recovery kit (Takara Bio Inc.). Bands were visualized using the GelDoc XR+ gel imaging system (Bio-Rad Laboratories, Inc.) through nucleic acid staining. Densitometric analysis was performed using Gel-Pro Analyzer 4.0 software (Media Cybernetics, Inc.).
- Protein expression of recombinant sus scrofa TFF3
B. choshinensis containing pNCMO2-TFF3-6xhis or pNCMO2 was grown in liquid MT medium containing 20 mg/l neomycin at 30°C for 60 h. Once the bacteria reached the logarithmic growth period at 37°C, isopropyl β-D-1-thiogalactopyranoside (final concentration 1 mmol/L) was added to the bacteria. After induction, the harvested bacteria were ultrasonically disrupted (JY88-II Ultrasonic Cell Disruptor; Bio-Equip) and lysed in PBS (pH = 7.4).
The bacterial pellet and supernatant were subsequently used in SDS-PAGE. Supernatants and cells were separated by centrifugation at 12,000 × g for 20 min at 4 °C. Proteins were detected by 14% reduction SDS-PAGE stained with Coomassie Brilliant Blue, and the amount of secreted protein was assessed. Gel-Pro Analyzer 4.0 software (Media Cybernetics, Inc.) was used to scan and measure band density on gels to assess the expression level of the target protein.
For Western blotting, protein samples were boiled in SDS/β-mercaptoethanol loading buffer (192 mM glycine and 25 mM Tris; pH 8.3; Beijing Dingguo Changsheng Biotechnology Co, Ltd.) and subsequently electrophoresed. A total of 10 µg protein/lane was separated by SDS-PAGE on a 14% gel. The proteins in the gel were transferred to PVDF membranes (Merck KGaA) by electrophoretic transfer. The membrane was then blocked for 1 h in 5% skim milk at room temperature. TBST [20 mM Tris (pH 8.0), 200 mM NaCl, and 0.1% Tween 20] was used to dilute mouse anti-6xhis-tag monoclonal primary antibody (Abcam; cat. no. ab18184; 1:2000).
The membrane was incubated overnight with the primary antibody at 4°C and washed three times with TBST for 15 min. Horseradish peroxide-conjugated secondary antibody (Abcam; goat-mouse IgG; cat. no. ab150113) was diluted 1:3000 with TBST and incubated with the membrane for 1 hour at room temperature. Subsequently, a wash was performed with TBST three times for 15 min. ECL development and X-ray film exposure methods were used to visualize the bands (Merck KGaA).